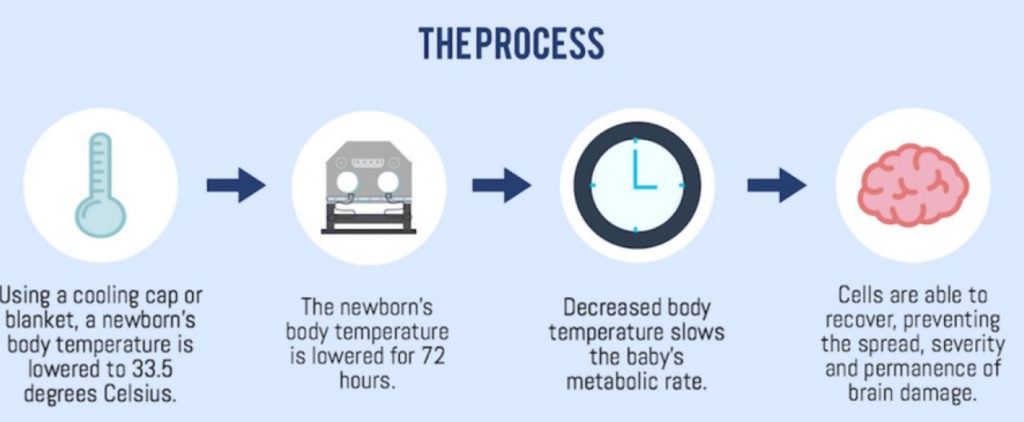
Therapeutic Hypothermia to the rescue of HIE-ridden patients.
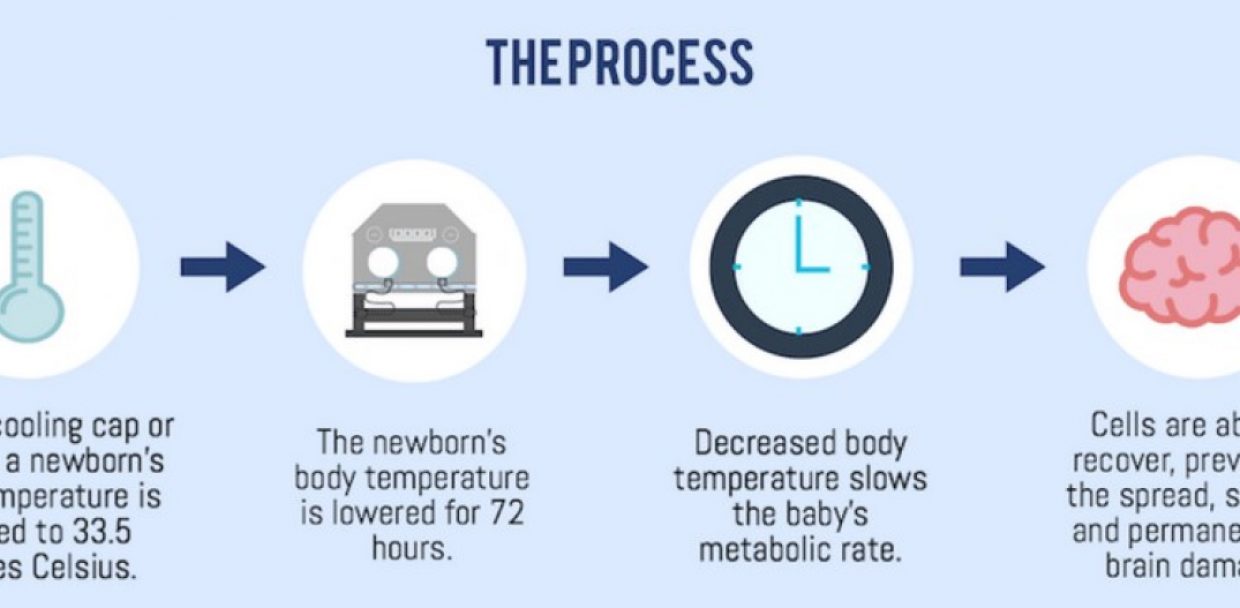
Reservations regarding the efficacy of Therapeutic Hypothermia in patients with HIE have been around since over a decade. Multiple large randomized controlled trials such as the notable TOBY trial, the NICHD trial, the CoolCap trial have been conducted to determine the efficacy of the treatment on patients with moderate and severe HIE along with determining the neurological outcome at 18 months of age. 18 months is considered the soonest age the assessment can be made using parameters such as the rate of severe disability, cerebral palsy, score of mental development and psychomotor indices of the Bayley scales and the quality of vision and hearing.
A meta-analysis was performed based on the outcomes of these three trials and published. The result of the analysis showed that the hypothermia treatment reduced the risk of mortality and disability in infants at 18 months of age. Secondary outcomes of rate of normal survival and with reduced risk of cerebral palsy were far higher for the treatment group than the control group. Similarly, rate of deafness was found to be 4.7% in the treatment group as compared to the 6% in the control group.
While babies with mild to moderate HIE showed promising results by the end of 18 months with Therapeutic Hypothermia treatment, the data for the same treatment in severe HIE cases is still inconclusive. The primary outcome, with the currently available data, unfortunately showed no significant improvement with TH treatment for severe HIE patients.