
Protecting the most Precious Brains - From Day One
When every second counts, we bring smart, cutting edge, life saving technology to protect the most vulnerable brains.
World's First and Only Whole Body Cooling with Integrated Brain Monitoring
REVIVE redefines neuroprotective care for newborns with Birth Asphyxia—combining therapeutic hypothermia and continuous aEEG monitoring in one breakthrough device, making life-saving therapy accessible across more hospitals.

Our Story
Every life begins with a moment of hope. But for some newborns, those first moments are fragile—shaped by complications they cannot fight on their own.
Sensivision Health Technologies Pvt. Ltd. was born from a simple yet powerful belief: no baby should lose their future because life-saving care was unavailable, unaffordable, or too complex to deliver.
In neonatal intensive care units, clinicians face heart-wrenching decisions every day. When a newborn suffers a lack of oxygen at birth, minutes matter. The right intervention, delivered at the right time, can mean the difference between a healthy childhood and a lifetime of challenges. Yet proven treatments often remain out of reach for many hospitals.
This is where Sensivision Health Technologies Pvt. Ltd. began.
Our first step was REVIVE, a therapeutic hypothermia system created to bring world-class neonatal care to settings where it was needed most. Designed hand-in-hand with doctors and nurses, REVIVE was built to be reliable, intuitive, and affordable—without compromising on safety or outcomes. Each baby treated reaffirmed our purpose.
But saving lives is only the beginning.
Today, Sensivision Health Technologies Pvt. Ltd. is moving toward a future where intelligence augments care—where subtle warning signs are detected earlier, where clinicians are supported by data-driven insights, and where every newborn benefits from the collective learning of many hospitals. Through AI-enabled monitoring and connected care platforms, we aim to give caregivers more clarity, confidence, and time—when it matters most.
We are guided by a simple promise: technology should serve compassion, not replace it.
As we look ahead, our mission remains unchanged—to ensure that every newborn, no matter where they are born, has a fighting chance at a healthy life.
Our Partners
Supported by leading institutions in innovation and healthcare










The Problem
Newborn Deaths Annually from Birth Asphyxia
A preventable tragedy affecting families worldwide
Global Cause
Birth Asphyxia ranks among the top 3 leading causes of newborn deaths worldwide
Survivors at Risk
Without proper treatment, survivors face lifelong disabilities
Lifelong Impact on Survivors
Without timely intervention, survivors face severe long-term disabilities
Cerebral Palsy
Motor function impairment
Mental Retardation
Cognitive development delays
Seizures
Recurring neurological episodes
Learning Disability
Educational challenges
Early intervention with therapeutic hypothermia can significantly reduce these outcomes
Traditional therapeutic hypothermia for HIE requires costly combinations of cooling systems and external patient monitors to track vitals—demanding skilled staff, multiple devices in sync, and complex management that most hospitals cannot provide.
Understanding Neonatal Brain Complications
Three major conditions that require immediate intervention and monitoring
Hypoxic-Ischemic Encephalopathy
Leading cause of neonatal brain injury in LMICs
Neonatal Seizures
Associated with poor neurodevelopment without monitoring
Perinatal Stroke
Lifelong motor and cognitive deficits

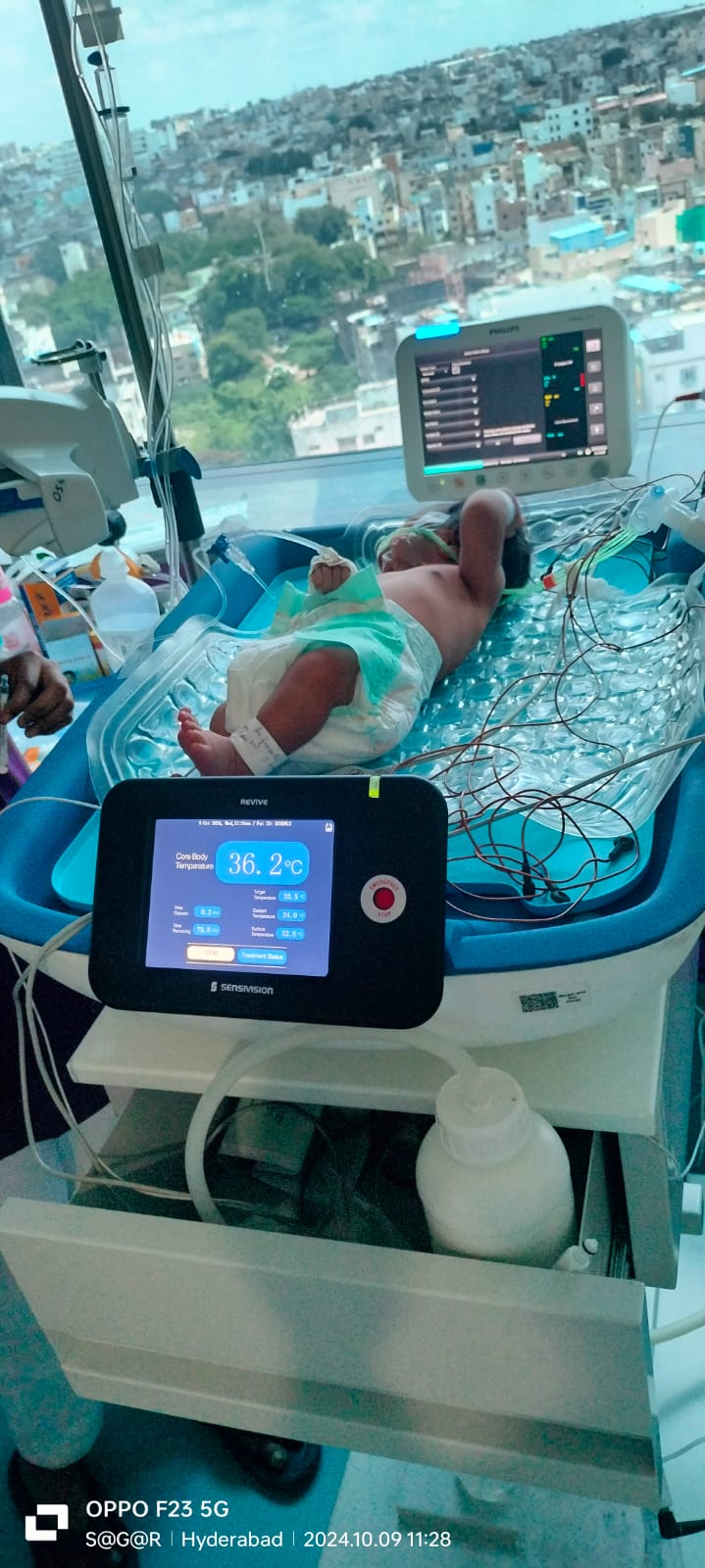
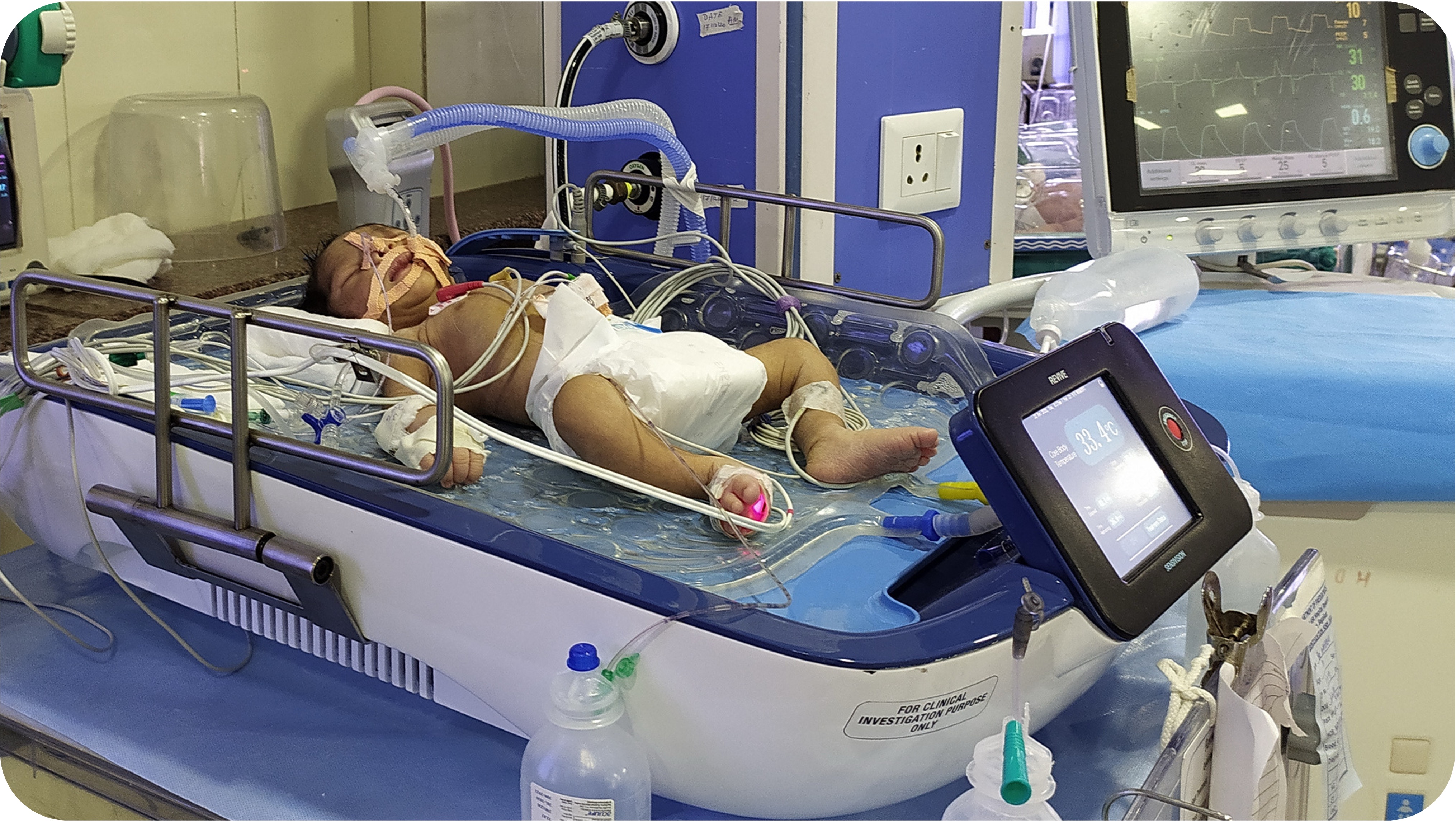
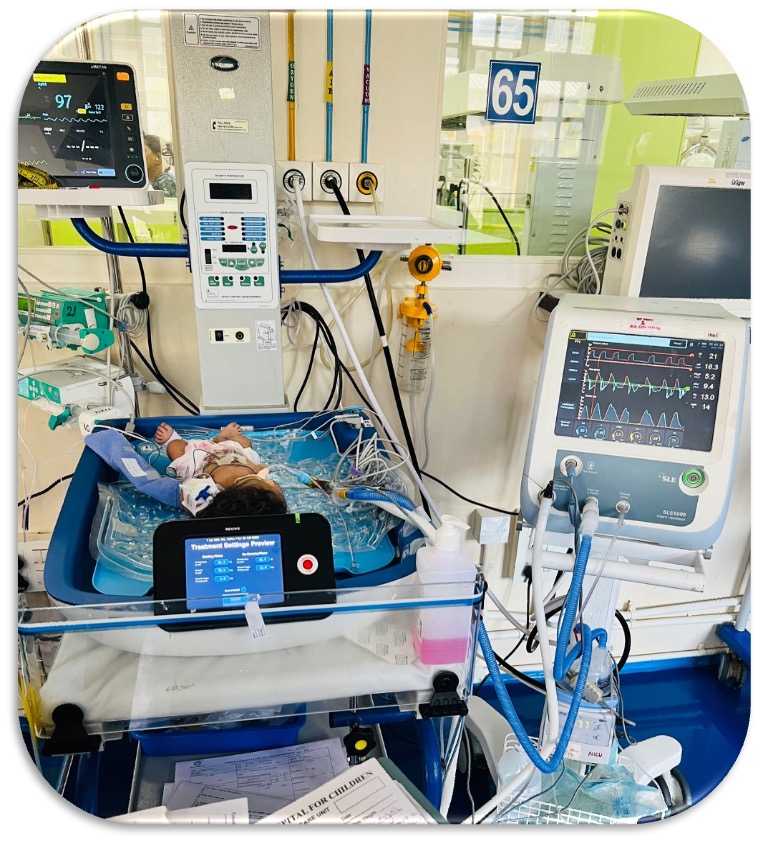
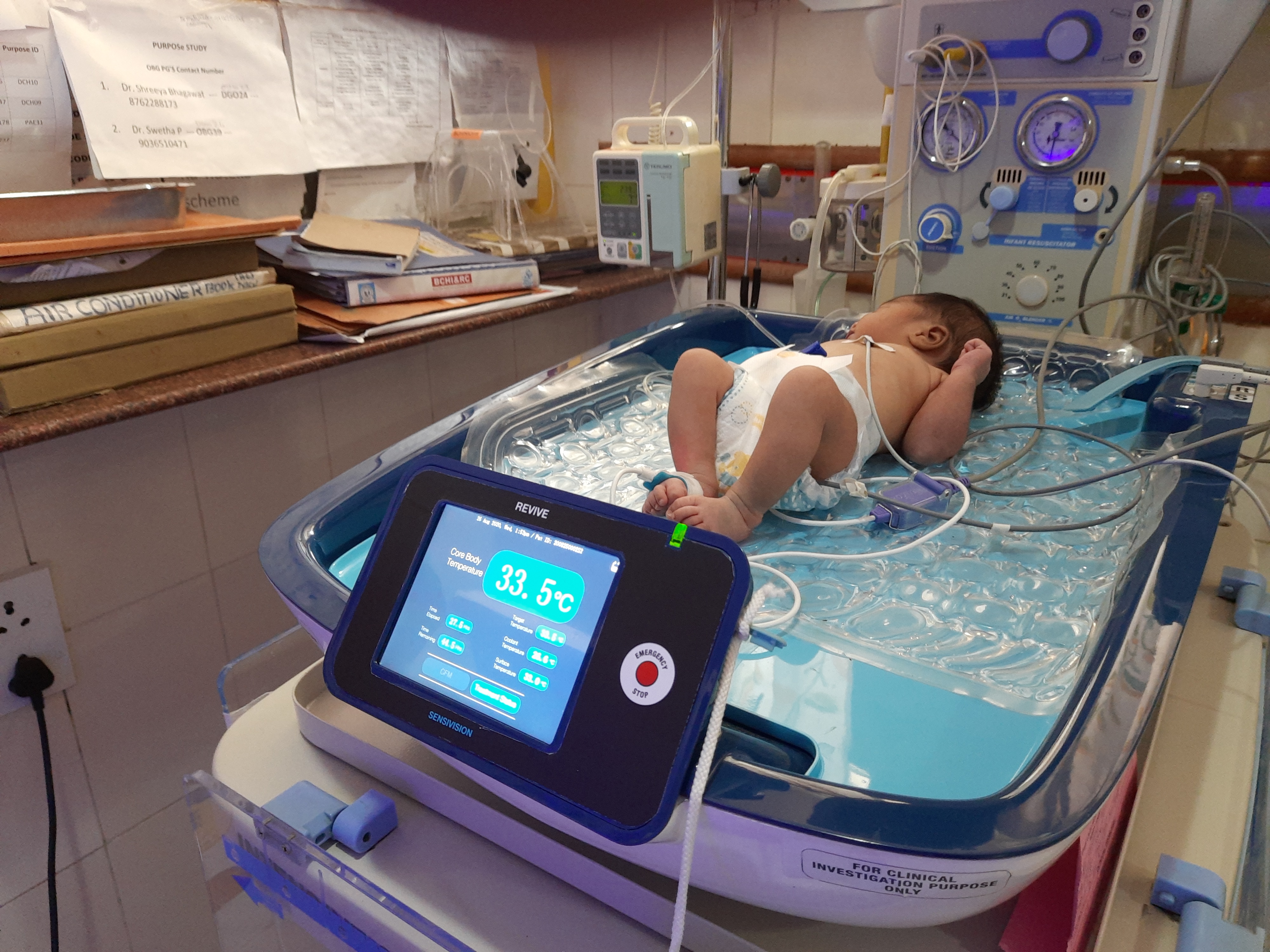
REVIVE: Revolutionary Whole Body Cooling with Brain Monitoring
REVIVE combines cutting-edge therapeutic hypothermia with real-time brain monitoring to deliver unprecedented outcomes in treating Hypoxic Ischemic Encephalopathy in newborns.
What is Unique About REVIVE

Integrated Brain Monitoring
World's first whole body cooling with built-in continuous aEEG monitoring
Real-Time Neurological Assessment
Continuous brain activity tracking during treatment for proactive intervention
Superior Clinical Outcomes
68% complication reduction with 40% faster recovery (8-12 days)
Automated Smart Feedback
Real-time vitals automatically adjust cooling, reducing staff burden and errors
Traditional Cooling Methods: Critical Limitations
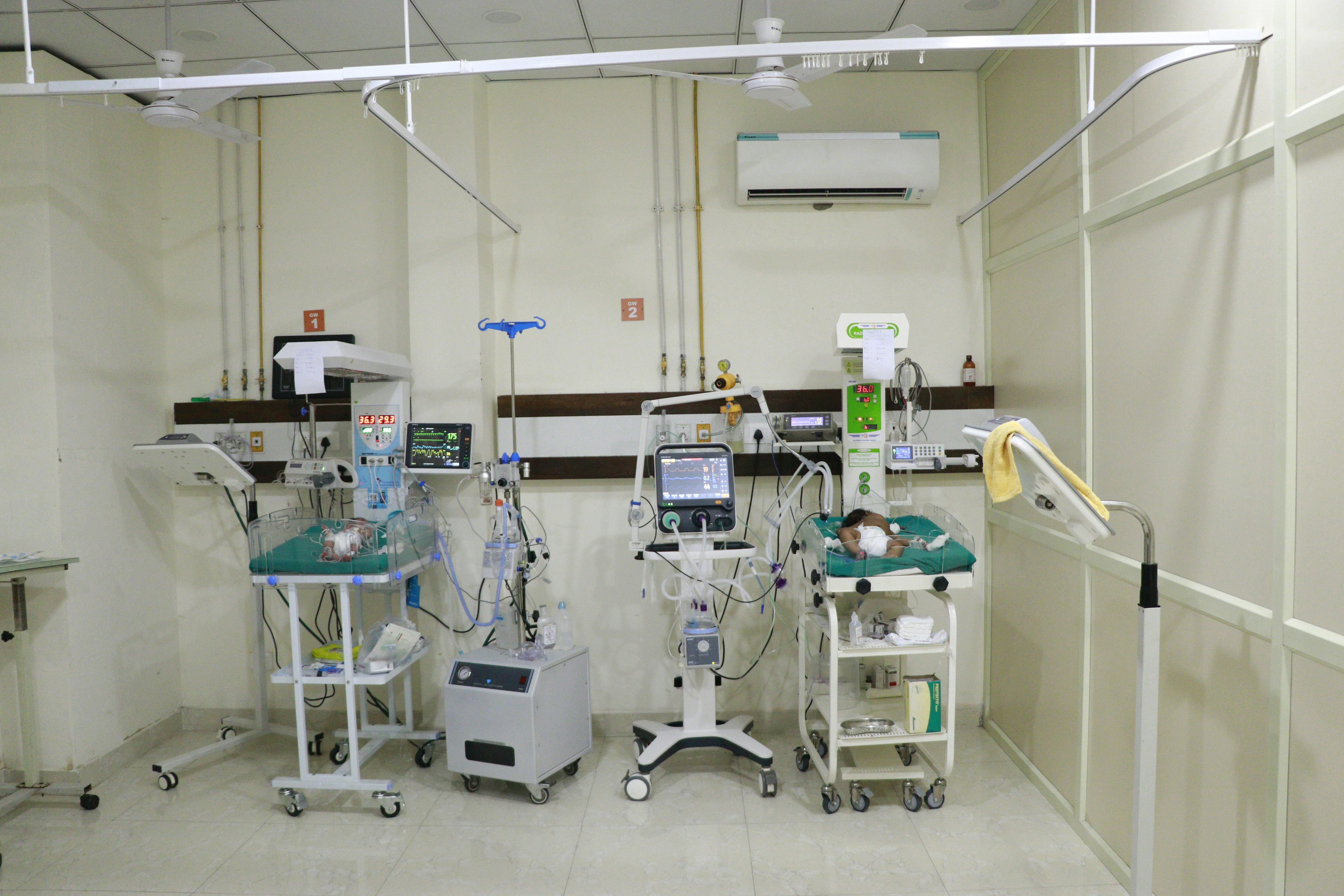
Limited Real-Time Monitoring
No continuous brain activity assessment during critical treatment window
Reactive Intervention
Complications detected only after they occur, missing prevention opportunities
Higher Complication Rates
32% complication reduction with longer 14-21 day recovery periods
Manual Monitoring Burden
Requires constant staff attention with periodic manual checks prone to human error
Clinical Advantage Comparison
| Feature | Traditional Methods | REVIVE System | Advantage |
|---|---|---|---|
| Treatment Efficacy | 32% complication reduction | 68% complication reduction | +36% improvement |
| Recovery Time | 14-21 days average | 8-12 days average | 40% faster |
| Monitoring Capability | Manual periodic checks | Real-time continuous monitoring | 24/7 precision |
| Rectal Probe slip detection | No detection | Automatic detection of slip | Safe, uninterrupted treatment |
Integrated Brain Monitoring
World's first system combining whole-body cooling with continuous built-in aEEG monitoring—eliminating multiple external monitors and reducing complexity, cost, and potential errors.
Smart Automated Feedback Loop
Real-time feedback from baby's vitals automatically adjusts cooling process, ensuring target therapeutic temperature is reached quickly and maintained safely while reducing NICU staff burden.
Resource-Constrained Design
Tailored for India and LMIC hospitals—cost-effective, portable, intuitive with built-in safeguards ensuring reliable operation even with inconsistent infrastructure or minimal training.
Data-Driven & Future-Ready
Built with AI integration in mind—vitals data supports algorithms for early detection of seizures or abnormal brain activity, setting the stage for comprehensive neonatal brain care platform.
Transformative Outcomes for Your NICU
Evidence-based results that demonstrate superior clinical performance across all key metrics that matter to neonatal care teams.
All-in-One System
REVIVE simplifies complex multi-device therapeutic hypothermia into one compact unit. Seamlessly combines whole-body cooling with continuous monitoring of core temperature, skin temperature, and aEEG—eliminating external monitors and reducing setup complexity.
Meet Our Team
The dedicated team behind REVIVE, committed to transforming neonatal care in India and beyond

Jayadeep Unni
Founder & CEO

Rohini Krishnamurthy
Director, Product & Manufacturing

Ashim Purohit
Chief Motivation Officer

Amala Nair
Product Manager

Cherian K.M
Sales South

Devang Lakhia
Sales West & North

Shjill Varghese
Design Engineer

Abhinav Shayish
Design Engineer

Shiva Sharma
Business Development Executive, North

Kavebharathi
Verification Engineer
Rigorous Clinical Evidence & Research
Our technology is validated through clinical trials in India, peer-reviewed research, and regulatory approvals from CDSCO and international medical authorities.
CDSCO Approved
License: MFGMD/2023/000619
ISO 13485 Certified
Medical Device Quality
Class II B Medical Device
EN ISO 13485:2016
Peer-Reviewed Publications
Therapeutic Hypothermia for Neonatal Encephalopathy in India: A Multi-Center Study
More K., Guruprasad G., Yelamali B., et al.
Integrated Brain Monitoring During Whole-Body Cooling in Resource-Limited Settings
Guruprasad G., More K., Sharma R., et al.
Cost-Effectiveness of Integrated Cooling Systems in Indian NICUs: 24-Month Follow-up
Yelamali B., Desai S., Patil M., et al.
Access Complete Clinical Evidence Library
Download comprehensive clinical trial data, peer-reviewed research, and regulatory documentation to support your evaluation process.
Product Specifications & Technical Advantages
Engineered with precision for the unique demands of neonatal care, combining cutting-edge technology with clinical practicality.

REVIVE System with in-built Brain monitoring

Complete REVIVE Therapeutic Hypothermia System in Clinical Use
Integrated Brain Monitoring
World's first whole body cooling device with in-built Amplitude Integrated EEG (aEEG) for real-time cerebral function monitoring during therapeutic hypothermia.
Precise Temperature Control
Configurable cooling range (30°C-35°C) with rapid cooling capability (30-60 min) and controlled re-warming (6-10 hours) for optimal neuroprotection.
NICU-Optimized Design
Compact, portable system with below 60dB noise level, NICU-friendly interface, and ambulance compatibility for seamless patient transport.
Comprehensive Data Management
Extensive data storage (~2000 hours) with USB 2.0 export capability for clinical documentation and treatment analysis.
Dimensions
722 x 507 x 220 mm
Weight
10-12 kg
Noise Level
Below 60 dB
Device Classification
Class B
Manufacturing License
MFGMD/2023/000619
Portability
Ambulance compatible
Request Detailed Technical Documentation
Download complete product specifications, technical manuals, and integration guides for your evaluation process.
Flexible Procurement Options for Your Institution
We offer multiple acquisition pathways designed to fit your hospital's budget and procurement processes in India and LMICs.
Equipment Lease
Flexible monthly leasing with full service and support included
- No upfront capital investment required
- Monthly payment structure fits operating budgets
- Includes all maintenance and software updates
- 24/7 clinical support and training
- Upgrade to latest technology every 3 years
- Tax-deductible operating expense
Direct Purchase
One-time purchase with comprehensive warranty and support
- Full ownership of equipment
- 5-year comprehensive warranty
- Lifetime software updates included
- Priority technical support
- Volume discount pricing for multi-site hospitals
- Flexible financing options available
Hybrid Model
Combination of purchase and per-use billing for maximum flexibility
- Lower upfront equipment cost
- Per-treatment usage fees
- Scales with patient volume
- Predictable monthly costs
- Full service and support included
- Ideal for growing NICUs in tier 2/3 cities
Procurement Process Timeline
Initial Consultation
1-2 daysSchedule a call with our medical device specialists to discuss your NICU needs and patient population.
Clinical Demo
1 weekOn-site demonstration with your clinical team, including hands-on training and Q&A session.
Proposal & Evaluation
2-3 weeksReceive customized pricing proposal and ROI analysis. Review with procurement and clinical teams.
Contract & Approval
2-4 weeksFinalize contract terms, complete institutional approval process, and schedule implementation.
Installation & Training
1-2 weeksProfessional installation, comprehensive staff training, and go-live support.
Total Implementation Timeline: 6-10 weeks
Your Questions Answered
Find answers to common questions about REVIVE technology, implementation, and clinical use.
Still Have Questions?
Our medical device specialists are available to answer your specific questions and provide detailed information about REVIVE implementation at your institution.
Every Day Matters in Neonatal Treatment
Reach out to our clinical team to explore how REVIVE can transform outcomes in your NICU.
Time-Sensitive
Every hour counts in neonatal brain injury. Early intervention with REVIVE maximizes outcomes.
Lives at Stake
150,000+ newborns face HIE annually in India. Your NICU could be saving more lives with REVIVE.
Proven Results
400+ neonates successfully treated. Join leading Indian hospitals already using REVIVE.
Connect With Our Clinical Team
Call Clinical Team
(+91) 9446065047
Available 24/7 for urgent inquiries
Email Clinical Team
contact@sensivisionhealth.com
Response within 4 business hours
Corporate Headquarters
Sensivision Health Technologies Pvt. Ltd, Bengaluru, Karnataka, India
Visit our office
Visit Our Office
Privacy & HIPAA Compliance Assurance
All communications and data handling comply with HIPAA regulations. Your institutional information and patient data are protected with enterprise-grade security. We maintain comprehensive Business Associate Agreements with all hospital partners.

Our Major Customers
Leading hospitals across India trust REVIVE for neonatal neuroprotective care
P.D Hinduja Hospital
Mumbai
Bai Jerbai Wadia Hospital for Children
Mumbai
Ankura Hospitals
Bhubaneshwar, Khammam
PGIMER
Chandigarh
KIMS Hospital
Secunderabad
Niloufer Hospital for Women and Children
Hyderabad
J.J.M Medical College & Hospital
Davangere
St. Johns Hospital
Bengaluru
GMERS Medical College & Hospital
Ahmedabad
KEM Hospital
Pune
Bharati Hospital
Pune
P.D Hinduja Hospital
Mumbai
Bai Jerbai Wadia Hospital for Children
Mumbai
Ankura Hospitals
Bhubaneshwar, Khammam
PGIMER
Chandigarh
KIMS Hospital
Secunderabad
Niloufer Hospital for Women and Children
Hyderabad
J.J.M Medical College & Hospital
Davangere
St. Johns Hospital
Bengaluru
GMERS Medical College & Hospital
Ahmedabad
KEM Hospital
Pune
Bharati Hospital
Pune
Clinicians Love REVIVE - "It Fits Seamlessly into Our NICU."
Hear directly from neonatologists and NICU directors at leading Indian hospitals about their experience with REVIVE technology.
Dr. Guruprasad G
MD, DCH, DNB, DM, FNNF, MNAMS, Professor, HOD Department of Neonatology
JJM Medical College, Davangere
Hospital Implementation Success Stories
A leading Children's hospital in Mumbai
Full NICU deployment - 18 beds
Implementation Results:
Neonates Successfully Treated
Leading Hospital Partners
Treatment Success Rate